Curis, Inc
Emavusertib (CA-4948) - Curis, Inc
June 26, 2024 - First-in-Class Suppressor of the TLR Pathway (Oral, Small Molecule IRAK4 Kinase Inhibitor) Innate immune responses orchestrated through Toll-like receptors or certain interleukin receptors are important mediators of the body’s initial defense against foreign antigens, while their dysregulation ...
Nih
Discovery of CA-4948, an Orally Bioavailable IRAK4 Inhibitor for ...
Small molecule potent IRAK4 inhibitors from a novel bicyclic heterocycle class were designed and synthesized based on hits identified from Aurigene’s compound library. The advanced lead compound, CA-4948, demonstrated good cellular activity in ABC ...
Ascopubs
Phase 1/2a study of the IRAK4 inhibitor CA-4948 as monotherapy ...
7016 Background: CA-4948 is a novel oral inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4) and FLT3. IRAK4 is critical in triggering inflammation, oncogenesis, and survival of cancer cells. Genetic mutations in the splicing factors SF3B1 and U2AF1 drive overexpression of a highly ...
Cancer
CA-4948 Added to Standard Chemotherapy to Treat ...
National Cancer Institute at the National Institutes of Health
Nih
Oral IRAK-4 Inhibitor CA-4948 Is Blood-Brain Barrier Penetrant ...
An ongoing challenge in cancer is the management of primary and metastatic brain malignancies. This is partly due to restrictions of the blood-brain barrier and their unique microenvironment. These challenges are most evident in cancers such as ...
Selleckchem
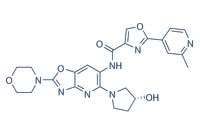
Emavusertib (CA-4948) | ≥99%(HPLC) | Selleck | IRAK inhibitor
January 17, 2022 - CA-4948 S-Isomer is the S-Isomer of CA-4948. CA-4948 is a potent and orally bioavailable inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK... Quality confirmed by NMR & HPLC. See customer reviews, validations & product citations.
Ascopubs
Open-label, dose-escalation, and expansion trial of CA ...
ASCO’s publications—including the Society’s flagship publication, the Journal of Clinical Oncology—serve readers as the most credible, authoritative, peer-reviewed resources for significant clinical oncology research that informs the delivery of high-quality patient care across the ...
OncLive
IRAK-4 Inhibitor CA-4948 May Enhance Immunotherapeutic Outcomes ...
May 23, 2023 - Bently Doonan, MD, discusses how CA-4948 can penetrate the blood-brain barrier and interact with the tumor microenvironment, and how this approach may open doors for patients with melanoma who have brain metastases.
Ufhealth
Oral IRAK-4 inhibitor CA-4948 in combination with Pembrolizumab ...
This phase I/II trial will investigate the use of the novel oral IRAK-4 inhibitor CA-4948 in combination with pembrolizumab therapy following stereotactic…
ScienceDirect

Safety, Pharmacokinetics and Activity of CA-4948, an IRAK4 Inhibitor, ...
IntroductionCA-4948 is a novel oral small molecule inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4). IRAK4 is part of the Myddosome sig…
Curis
Safety, Pharmacokinetics and Activity of CA-4948, an IRAK4
About Curis is a biotechnology company focused on the development and commercialization of innovative therapeutics for the treatment of cancer. We currently have three drug candidates in development: Fimepinostat (formerly known as CUDC-907), an orally available, small molecule inhibitor of ...
Ashpublications
Safety, Pharmacokinetics and Activity of CA-4948, an IRAK4 Inhibitor, ...
November 5, 2020 - IntroductionCA-4948 is a novel oral small molecule inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4). IRAK4 is part of the Myddosome signalin
Caymanchem
CA-4948 (CAS 1801343-74-7)
CA-4948: An IRAK-4 inhibitor. CAS Number: 1801343-74-7. Purity: ≥98%.
Mskcc
A Study of CA-4948 in Patients with Recurrent or Persistent ...
Full Title An Open-Label, Dose Escalation and Dose Expansion Trial Evaluating the Safety, Pharmacokinetics, Pharmacodynamics, and Clinical Activity of Orally Administered CA-4948 in Patients with Relapsed or Refractory Primary Central Nervous System Lymphoma Purpose The purpose of this study ...
Aacrjournals
Abstract P243: The IRAK4 inhibitor CA-4948 demonstrates antitumor ...
December 1, 2021 - CA-4948 is an oral first-in-class small molecule inhibitor of IRAK4 that has demonstrated clinical activity in patients with systemic Non-Hodgkin’s Lymphoma. Our study goal was to evaluate CA-4948 efficacy in a syngeneic preclinical model of primary CNS B cell lymphoma (A20), supporting future ...
Confex
Paper: Preliminary Safety and Efficacy of Emavusertib (CA-4948) ...
December 10, 2023 - The safety, clinical activity, ... (NCT04278768). Here we present preliminary safety and efficacy data in the subset of enrolled AML patients who carried FLT3 mutation (FLT3m) at baseline and were treated with emavusertib monotherapy....
Medkoo
CA-4948-Analog | CAS#1801343-74-7 | IRAK4 inhibitor | MedKoo
CA4948-Analog is an analog of Emavusertib. It has similar property to CA-4948, which is a potent, and orally active interleukin-1 receptor-associated kinase 4 (IRAK4) inhibitor. It was reported in patent WO 2015104688. Emavusertib, also known as CA-4948 is a potent IRAK4/FLT3 inhibitor with ...
Aacrjournals
Abstract P073: Pharmacological inhibition of IRAK4 with CA-4948 ...
December 1, 2021 - IRAK4 is a protein kinase downstream the Toll-like receptor signaling. CA-4948 is the first-in-class inhibitor of IRAK4 and it is in phase 1/2 studies for patients with relapsed/refractory clinical studies with favorable activity in lymphomas and myeloid neoplasms.
Aacrjournals
Abstract 127: Efficacy of novel IRAK4 inhibitor CA4948 in AML and ...
July 1, 2017 - To functionally determine the role of IRAK4 in MDS/AML pathogenesis, we utilized CA-4948, a potent, oral, small-molecule inhibitor of IRAK4, to assess the effect of inhibiting IRAK4 catalytic activity. In vitro, CA-4948 blocked downstream NF-κB pathway signaling, including secretion of ...